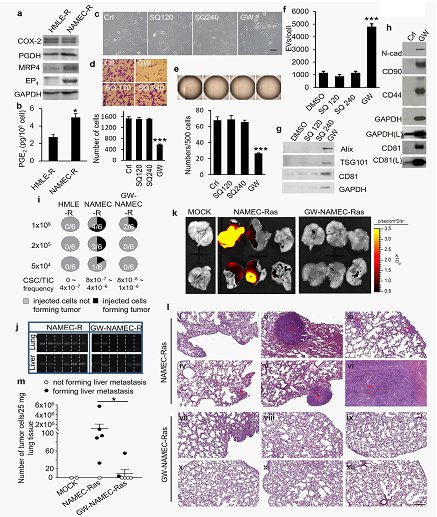
Cells expressing mesenchymal/basal phenotypes in tumors have been associated with stem cell properties, while cancer stem cells (CSCs) are often resistant to conventional chemotherapy. A team led by Dr. Hua-Jung Li from the Institute of Cellular and System Medicine, explored overcoming mesenchymal CSC resistance to chemotherapeutic agents with the goal of reducing CSC numbers in vivo, in conjunction with chemotherapy to reduce tumor burden.
Analysis of clinical samples demonstrated that COX-2/PGE2 /EP4 signaling is elevated in basal-like and chemoresistant breast carcinoma and is correlated with survival and relapse of breast cancer. EP4 antagonism elicits a striking shift of breast cancer cells from a mesenchymal/CSC state to a more epithelial non-CSC state. The transition was mediated by EP4 antagonist-induced extracellular vesicles [(EVs)/exosomes] which removed CSC markers, mesenchymal markers, integrins, and drug efflux transporters from the CSCs. In addition, the team found that EP4 antagonism-induced CSC EVs/exosomes can convert tumor epithelial/non-CSCs to mesenchymal/CSCs enabling to give rise to tumors and to promote tumor cell dissemination. Because of its ability to induce a CSC-to-non-CSC transition, EP4 antagonist treatment in vivo reduced the numbers of CSCs within tumors and increased tumor chemosensitivity. This study demonstrates EP4 antagonist treatment enhances tumor response to chemotherapy by reducing the numbers of chemotherapy-resistant CSCs available to repopulate the tumor. EP4 antagonism can collaborate with conventional chemotherapy to reduce tumor burden. The finding has recently been published in International Journal of Cancer (2018 Apr 15;Article in Press http://dx.doi.org/10.1002/ijc.31523).
