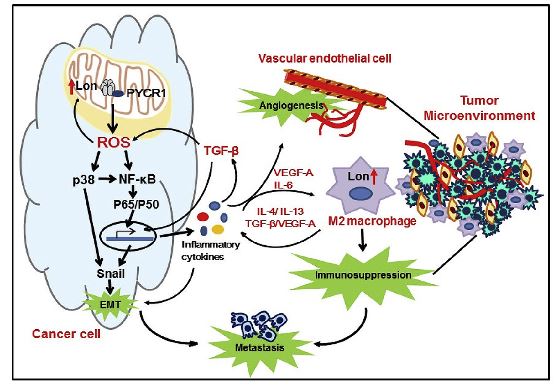
 Chronic inflammation has been linked to cancer progression and metastasis. Reactive oxygen species (ROS) induce inflammatory responses, and prolonged ROS production is considered to lead to chronic inflammation. Mitochondria are a major source of ROS production in a cell. However, the mechanism of how mitochondrial ROS by Lon regulates cancer metastasis in the tumor microenvironment (TME) remains unclear. Dr. Alan Yueh-Luen Lee and team from the National Institute of Cancer Research identified pyrroline-5-carboxylate reductase 1 (PYCR1) as a client of chaperone Lon, which induces mitochondrial ROS by Lon.
Chronic inflammation has been linked to cancer progression and metastasis. Reactive oxygen species (ROS) induce inflammatory responses, and prolonged ROS production is considered to lead to chronic inflammation. Mitochondria are a major source of ROS production in a cell. However, the mechanism of how mitochondrial ROS by Lon regulates cancer metastasis in the tumor microenvironment (TME) remains unclear. Dr. Alan Yueh-Luen Lee and team from the National Institute of Cancer Research identified pyrroline-5-carboxylate reductase 1 (PYCR1) as a client of chaperone Lon, which induces mitochondrial ROS by Lon.
The team further demonstrated that upregulation of mitochondrial Lon induces epithelial-mesenchymal transition (EMT) and angiogenesis through mitochondrial ROS-dependent p38-NF-κB pathways. Mitochondrial Lon induced ROS-dependent production of inflammatory cytokines, such as TGF-β, IL-6, IL-13, and VEGF-A, which consequently activated EMT, angiogenesis, and M2 macrophage polarization. In addition, Lon expression was induced upon the activation and M2 polarization of macrophages, which further promoted M2 macrophages to enhance the immunosuppressive microenvironment and metastatic behaviors in the TME. These findings raised the possibility that manipulation of the mitochondrial redox balance in the TME may serve as a therapeutic strategy to improve T cell function in cancer immunotherapy. The above findings have been published in Cancer Letters (2020 Jan 24;474:138-150).
Citation: Kuo, CL; Chou, HY; Chiu, YC; Cheng, AN; Fan, CC; Chang, YN; Chen, CH; Jiang, SS; Chen, NJ; Lee, AYL. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Letters (2020 Jan 24;474:138-150).
