A team led by Dr. B. Lin-Ju Yen from the Institute of Cellular and System Medicine, in collaboration with Dr. Ko-Jiunn Liu, National Institute of Cancer Research and Vice President Dr. Huey-Kang Sytwu, from National Institute of Infectious Diseases and Vaccinology, recently published their findings in Stem Cells (2018 Feb 3. doi: 10.1002/stem.2795), supporting that iPSC‐MSCs possess low oncogenicity and strong immunomodulatory properties regardless of cell‐of‐origin or reprogramming method and are good potential candidates for therapeutic use.
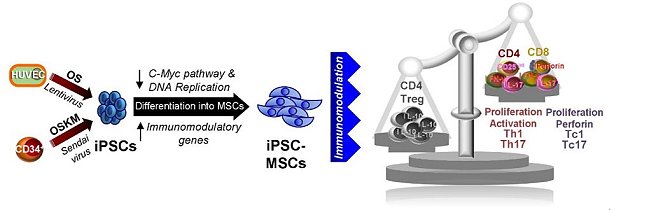
Multilineage tissue‐source mesenchymal stem cells (MSCs) possess strong immunomodulatory properties and are excellent therapeutic agents, but require constant isolation from donors to combat replicative senescence. The differentiation of human induced pluripotent stem cells (iPSCs) into MSCs offers a renewable source of MSCs; however, reports on their immunomodulatory capacity have been discrepant. Using MSCs differentiated from iPSCs reprogrammed using diverse cell types and protocols, and in comparison to human embryonic stem cell (ESC)‐MSCs and bone marrow (BM)‐MSCs, the team performed transcriptome analyses and assessed for functional immunomodulatory properties.
Differentiation of MSCs from iPSCs results in decreased c‐Myc expression and its downstream pathway along with a concomitant downregulation in the DNA replication pathway. All four lines of iPSC‐MSCs can significantly suppress in vitro activated human peripheral blood mononuclear cell (PBMC) proliferation to a similar degree as ESC‐MSCs and BM‐MSCs, and modulate CD4 T lymphocyte fate from a type 1 helper T cell (Th1) and IL‐17A‐expressing (Th17) cell fate to a regulatory T cell (Treg) phenotype. Moreover, iPSC‐MSCs significantly suppress cytotoxic CD8 T proliferation, activation, and differentiation into type 1 cytotoxic T (Tc1) and IL‐17‐expressing CD8 T (Tc17) cells. Coculture of activated PBMCs with human iPSC‐MSCs results in an overall shift of secreted cytokine profile from a pro‐inflammatory environment to a more immunotolerant milieu. iPSC‐MSC immunomodulation was also validated in vivo in a mouse model of induced inflammation, in which Th1/Th17 and Tc1/Tc17 responses were suppressed, and Tregs responses were enhanced. Overall, these results strongly support iPSC-MSCs as a viable source for therapeutic use towards regenerative medicine as well as immune-related diseases.
Citation: Wang, LT; Jiang, SS; Ting, CH ; Hsu, PJ; Chang, CC; Sytwu, HK; Liu, KJ; Yen, BL. Differentiation of mesenchymal stem cells from human induced pluripotent stem cells results in downregulation of c‐Myc and DNA replication pathways with immunomodulation toward CD4 and CD8 cells.
http://dx.doi.org/10.1002/stem.2795 Stem Cell. 2018 Feb 3; Article in Press.
